Classify the Following Phase Changes as Endothermic or Exothermic
A Calculate the minimum frequency of the radiation. On the other hand an exothermic reaction releases energy into the surrounding of the system.
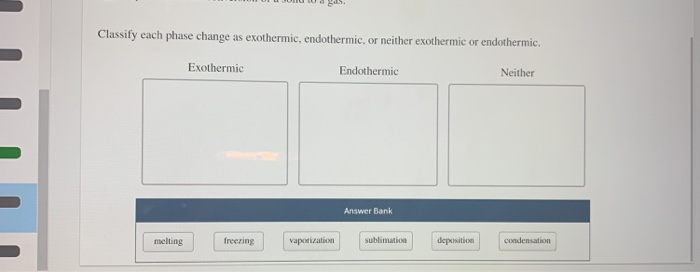
Solved Om A Ds Classify Each Phase Change As Exothermic Chegg Com
Classify each of the following processes as exothermic or endothermic and indicate the sign of ÎH butane gas burning in a lighter the burning of wax in a candle aexothermic Î H bendothermic Î H cexothermic -Î H dendothermic -Î H.
. N2 3H2 right arrow 2NH3 2S 3O2 right arrow 2SO3. Evaporation of sea water 4. All phase changes are accompanied by changes in the energy of a system.
When energy is released in an exothermic reaction the temperature of the reaction mixture increases. The stronger the forces the more heat energy is needed to overcome them. For example melting of ice is an endothermic phase change.
A process is called endothermic when it absorbs energy from surrounding. Write your answer on your answer sheet. They include melting sublimation and boiling.
Classify the following phase changes as exothermic processes or endothermic processes Studytuts General. A popular example of an endothermic chemical reaction is photosynthesis. Give reasons for your classifications.
Classify the following as physical or chemical changes and exothermic or endothermic processes. The correct answer is B Vaporization. These changes take up energy from the surroundings usually in the form of heat.
Freezing when a liquid transforms to solid. Endothermic phase changes take in heat from the surrounding environment. Energy is to be supplied for this process and therefore it is an endothermic process.
Deposition condensing freezing endothermic process. The forces that bind together the atoms and molecules of a given substance determine its melting and boiling points. The change is endothermic meaning that the system absorbs energy on going from solid to liquid.
In vaporization energy is absorbed by water molecules which. The change is exothermic the system releases energy when the direction is from liquid to solid. When energy is absorbed in an endothermic reaction the temperature decreases.
The following are exothermic phase changes. 1 For the given reactions classify the reactants as the reducing agent oxidizing agent or neither. We review their content and use your feedback to keep the quality high.
Experts are tested by Chegg as specialists in their subject area. Latent Heat of Vaporization. Phase changes are of two types.
Changes of state are examples of phase changes or phase transitions. Answer- Endothermic. In simple terms the endothermic reactions absorb energy from the surrounding that is in the form of heat.
Form of latent heat where energy flow during the change of phase is from solid to liquid. For a clear diffraction pattern to be seen from a regularly spaced lattice the radiation falling on the lattice must have a wavelength that is less than the lattice spacing. It involves the change of solid state ice to the liquid state water.
This question las luipe palls. During this process plants absorb energy from the Sun and convert it into. Burning of candle 5.
The length of a unit cell of NaCl is 566 pm. When any process releases energy in the surrounding then it is called exothermic process. Ozone layer formation B.
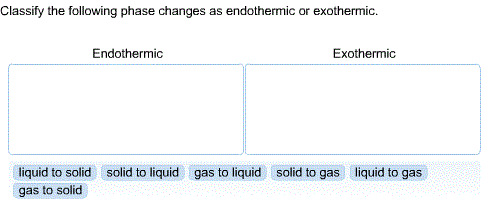
An endothermic phase change is when the substance absorbs energy from its surroundings melting vaporizationIn an exothermic phase change the substance releases energy to its surroundings. There are two situational examples. 1 solid to liquid 2 solid to gas 3 liquid to gas Exothermic.
In other words if energy is consumed in any process then it is termed endothermic change. 100 136 ratings Transcribed image text. 1 gas to li View the full answer.
Identifying Exothermic Endothermic Reactions. Freezing condensing deposition Endothermic Process. EXOTHERMIC AND ENDOTHERMIC REACTIONS IN OUR LIVES Directions.
This is the energy that is absorbed. In endothermic phase changes intermolecular binds are broken requiring energy. Drag the appropriate items to their respective bins.
Direction classify each of the following changes as. Classify the following phase changes as exothermic processes or endothermic processes. There are two methods for distinguishing between exothermic and endothermic reactions.
Condensation when a gas transforms to liquid. Classify each of the following changes as exothermic or endothermic. In exothermic phase changes molecules bind to each other in a formation that requires less.
The Home of All Assignment and Homework. Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes.

Solved Classify The Following Phase Changes As Endothermic Chegg Com

Learn About Endothermic And Exothermic Reactions The Fun Way Make Cold And Hot Chemical Volcanoes Using Safe Househo Exothermic Reaction Volcano Projects Cold
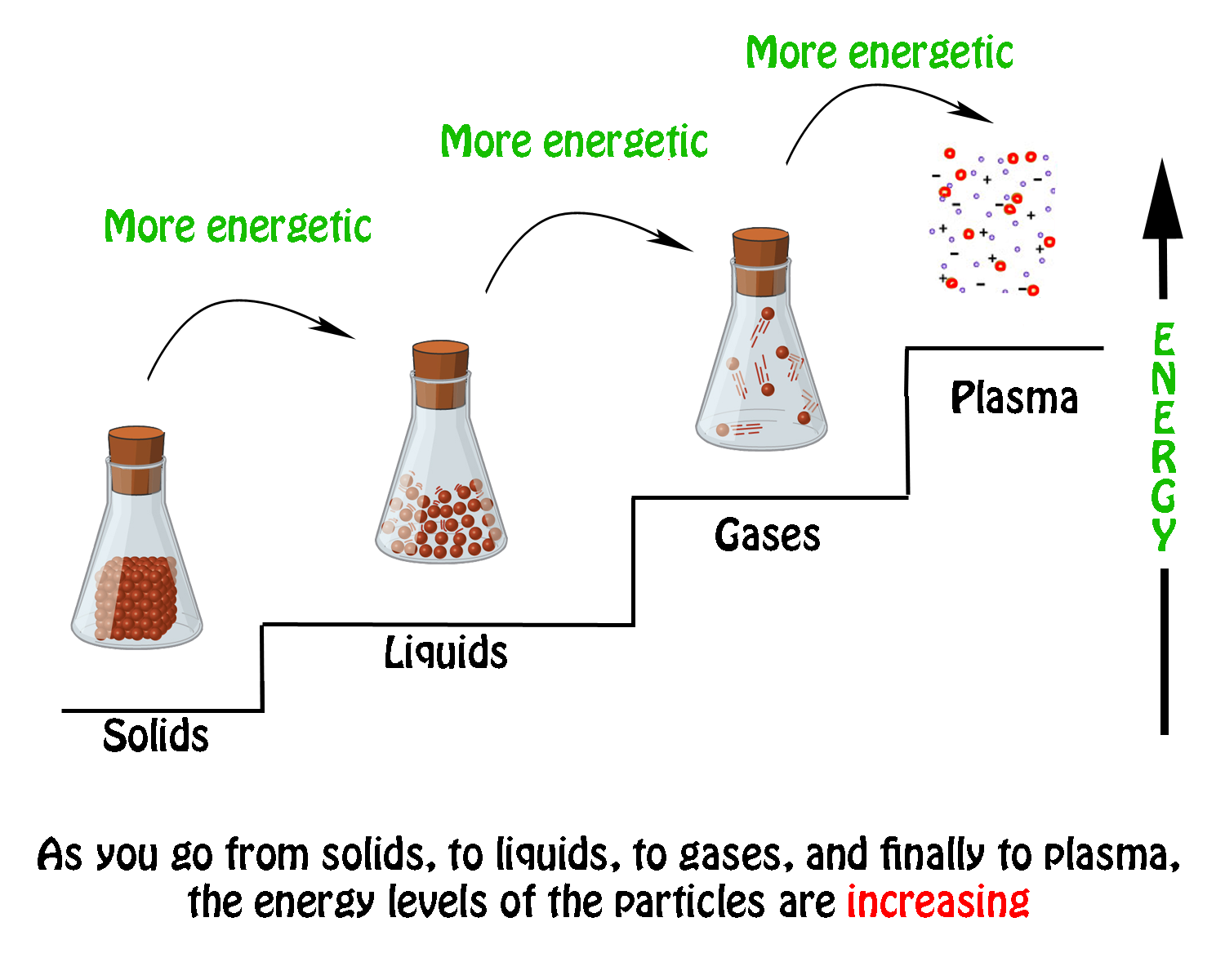
For The Three States Of Matter Solid Liquid And Gas There Are Six Possible Changes Of State Which Changes Of State Are Exothermic And Which Are Endothermic Socratic
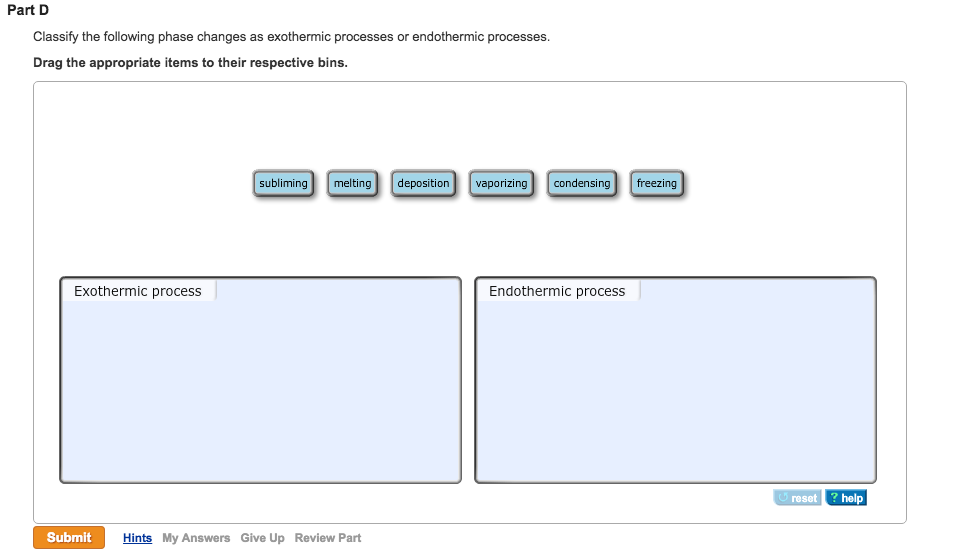
Solved Classify The Following Phase Changes As Exothermic Chegg Com
No comments for "Classify the Following Phase Changes as Endothermic or Exothermic"
Post a Comment